Rice sheath blight disease (cancer) has caused major crop failures worldwide. Management of the pathogen Rhizoctonia solani is difficult because of the wide host range and sclerotia formation that can persist under harsh environmental conditions; thus developing improved disease management practices without the use of toxic chemicals has been viewed as a key concern for sustaining sustainable agriculture. This presented study revealed the negative effects of nano silver (SNPs) on R. solani and disease progression both in vitro and in vivo. The side effects of SNP for R. solaniph depend significantly on the number of SNPs, sprayed at different concentrations in vitro. The highest inhibitory levels for sclerotia formation and mycelium growth were 92 and 85% respectively at a SNPs concentration of 50 ppm. In vivo greenhouse experiments also showed that SNP at the same concentration had a beneficial effect on both fresh and dry weight of rice plants with a significant inhibitory effect on leaf pathogen development.
Nội dung bài viết
Introduce
The application of nanomaterials has widely influenced drug delivery, cancer treatment [1], energy [2], biomedical [3], agriculture [4] and many other high-tech industries in the industry. in recent years [5].
Nanotechnology has led to new ways of controlling disease using atom-scale materials [6, 7]. The microscopic particles have emerged as modern agents due to their large surface-to-volume ratio, creating a large contact surface with pathogens [8]. Nanotechnology can have a major impact on natural and agricultural processes by introducing small-scale tools [9]; plant protection drugs [10]; fertilizer [11]; water filtration and pollution treatment [12]; nanosensors, diagnostic equipment [13]; and genetically modified plants [14]. Among nanoparticles (NPs), silver NP (SNP) can attack microorganisms, including cell membrane structures, in large-scale biological processes [15, 16]. The antibacterial activity of silver ions is well established and due to the ability of ionized SNPs to penetrate the bacterial cell wall and modulate cell signaling [17]. SNPs with antifungal, bacteriostatic and plasmonic properties are one of the more environmentally friendly inhibitors of plant pathogens compared with synthetic fungicides [18]; however, the antifungal ability of nano silver is less noticeable than in medical and pharmaceutical science with only a few studies being done against phytopathogenic fungi such as Alternaria Alternata, Botrytis cinerea [19] and Colletotrichum. gloeosporioides [20].
Rice (Oryza sativa L.) is the staple food for a large proportion of the world’s population, and is an important staple crop in muddy agricultural lands [21]. The blight caused by Rhizoctonia solani Kühn AG1 (Teleomorph: Thanatephorus cucumeris; anastomosis group 1 IA, AG1 IA) is a common rice disease in all rice growing regions of the world. The germination of sclerotia is a major factor in the spread of the sclerotia in rice, so any potential inhibitors of the germination of sclerotia, i.e. SNPs, would be essential. to reduce pathogens. This forces rice farmers to use large amounts of anti-natural chemicals and harmful chemicals every year to prevent arid, which not only adds costs in the short term, but also increases losses in terms of damage. long term for human health and the environment. In this study, to control rice blight with an emphasis on cleaner production at a lower cost, different silver nanoscale concentrations were tested as a novel antifungal agent to suppress inducing activity. disease of R. solani in vitro (to evaluate the inhibitory effect of SNP on mycelium formation and mycelium development) and in vivo (to investigate the effect of antifungal activity of nano silver on rice plants under greenhouse test conditions).
Materials and methods
1 Reagents, grains and sources of pathogens
Suspensions of SNPs were obtained from Nanocide Co., Tehran, with a concentration of 4000 ppm and an average particle size of 5–10 nm, in the form of a dark brown colloidal material. Rice seeds O. sativa L. var Hashemi and pure cultures of R. solani AG-1 IA were obtained from the Iranian Rice Research Institute (IRRI), Rasht [22]. O. sativa L. var Hashemi is capable of high yield but susceptible to dry disease. The fungus was maintained on potato dextrose agar (PDA, Merck Co.) at room temperature.
2 In vitro examination of the inhibitory effect of SNPs on the mycelium and sclerotia of R. solani
To assess the in vitro antifungal effect of nano silver against R. solani AG1, four different concentrations of the SNPs suspension (5, 10, 25 and 50 ppm) were added to Petri dishes prior to pouring with PDA. . Homogeneous agar nodes of 6 mm diameter containing mycelia were incubated simultaneously in the center of each Petri dish containing SNP, then incubated at 28 ± 1 ° C for three days. The growth inhibition rate of mycelium was calculated using (1) [4].
Inhibition rate (RH%) = (R-r) / R * 100%
The parameter RH is the inhibition rate, R for the mycelium inhibition growth is the diameter expansion of the mycelium in the control plate (cm), and for the growth of sclerotia during inhibition. , R is the weight of the sclerotia in the control dish (mg). The parameter r for the mycelium inhibition development is the expansion of the mycelium when treated with SNP (cm), and for the sclerotia development during inhibition, r is the weight. of sclerotia when treated with SNPs (mg). The antifungal effect of SNPs against R. solani sclerotia formation was measured after adding four nano-silver concentrations to PDA medium content. Inoculated R. solani plates were maintained at room temperature for two weeks to exhibit sclerotia, and the inhibition rate of sclerotia was calculated using (1). All tests were performed in three times. The effect of SNP on the germination of sclerotia was tested using the following procedure [23]. Fungal nodules of R. solani were formed on PDA at 15 ° C through incubation of inoculated plates for one week. Homogeneous sclerotia were collected from PDA plates, and the surface was sterilized in 1.5% sodium hypochlorite solution for 3 minutes. Three surface sterilized sclerotia were then treated with SNP at different concentrations and placed in Petri dishes, which were then incubated for one week at 25 ° C in the dark. The germination rates of the sclerotia were measured and compared with the control

Figure 1 Inhibitory effects of different SNP concentrations (shown at top right of left column) on R. solani AG1 Mycelium growth stage [left column (a) – ( e)], sclerotia [middle column (f) – (j)], Scion germination [right column (k) – (o)]
3 Examination of nano silver in arid under greenhouse conditions
Rice seeds are sown 3–4 cm below the soil surface of the pot (1 L) and they are divided into six groups with four pots in each group as follows: (a) single pathogens, (b) pathogens + SNPs (5 ppm), (c) pathogens + SNPs (10 ppm), (d) pathogens + SNPs (25 ppm), (e) pathogens + SNPs (50 ppm) and (f) controls (no SNPs) . Rice plants are potted in a greenhouse at 30 ° C and relative humidity is 85–95%. When the plants reached the late tillering stage (three-week old plants), they were treated with the R. solani fungal culture process. To achieve this, R. solani mycelium suspension (5 × 108 CFU / ml) was sprayed evenly with a hand spray on rice plants [24]. To maintain a reasonable coverage of nano-silver in the foliage throughout the evaluation period, two sprays were applied including 24 hours after transplanting and 7 days thereafter. After spraying with anti-pathogen and nano-silver spray, seedlings were covered with a plastic bag for three days to maintain high humidity. After 15 days, the severity of the disease was recorded by measuring fresh weight, dry weight and relative injury height (RLH), according to the 1996 IRRI standard. use (2) [25]
RLH% = [Lesion height (cm) / Plant height (cm)] * 100%
4 Statistical analysis
Recorded data were analyzed for variance using SAS software (SAS Institute, version 9). Testing Duncan ‘s range was used to compare vehicles.
Result
1. In vitro tests for inhibitory effects of SNP on the mycelium and sclerotia of R. solani
The effects of the SNPS concentration tested on mycelium growth, sclerotia formation and germination are shown in Figure 1. The plates treated with 50 ppm SNPs show dark sclerotia counts. minimal. RHs 12, 23, 68 and 92% were found to correspond to concentrations of SNPs 5, 10, 25 and 50. Concerning mycelium growth, RHs of 8, 35, 67 and 85 have been recorded for The SNPs of the concentrations are 5, 10, 25 and 50 ppm, respectively. RHs of 15, 26, 57 and 98% were found for SNPs concentrations of 5, 10, 25 and 50 ppm associated with sclerotia germination. These results indicated that SNPs strongly inhibited R. solani under in vitro conditions (Figure 2).
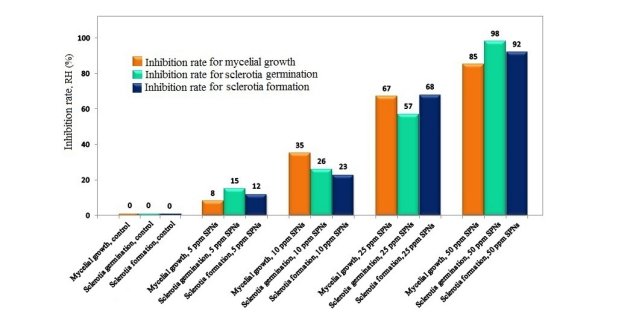
Figure 2 RH in vitro of nano silver affects mycelium growth, mycelium germination and sclerotia formation of R. solani AG1-IA
2 In vivo test for blight under greenhouse conditions
Figure 3 shows rice at 90 days late tillering under greenhouse conditions (30 ° C, humidity 85–95%). In vivo results of SNP against R. solani, the agent that causes blight, are shown in Figure 4. Figure 4a shows foliar symptoms caused by R. solani infection and Fig. 4b – e indicates pathogens plus 5, 10, 25 and 50 ppm SNPs applied and their different degrees of inhibition of pathogenesis. Treating the plants with the pathogen without SNPs resulted in typical arid symptoms, but plants treated with the pathogen + SNPs showed different degrees of inhibitory effect. According to the analysis of variance of different traits, all traits are different at significance level P ≤ 0.05. Significantly reduced symptoms of pathogens in nano-silver treated pots. The concentration of SNPs 5 ppm had a small effect on the dry weight of rice plants (Table 1); By increasing the concentration of SNPs by 50 ppm, the fresh and dry masses are significantly increased. At the SNPs concentration of 50 ppm, the RLH of each tiller decreased, evidence suggests that the application of silver nanoparticles had a strong antifungal effect and minimized injury in tillering paddy. The comparative results of the inhibitory activity of nano silver against blight showed a significant reduction of lesions on arid (Figure 5).

Figure 3 Rice seedlings after 90 days under greenhouse conditions, with temperature 30 ° C and constant humidity 85–95%

Figure 4 Effects of SNP treatment on pathogenesis of R. solani on rice leaves (a) Pathogen alone, (b) Pathogen + SNPs (5 ppm), (c) Pathogen + SNPs (10 ppm), (d) Pathogen + SNPs (25 ppm), (e) Pathogen + SNPs (50 ppm), (f) Untreated controls

Figure 5 Effect of SNP on pathogenesis of R. solani on dry shit (a) Pathogen alone, (b) Pathogen + SNPs (5 ppm), (c) Pathogen + SNPs (10 ppm), (d) Pathogen + SNPs (25 ppm), (e) Pathogen + SNPs (50 ppm), (f) Untreated controls
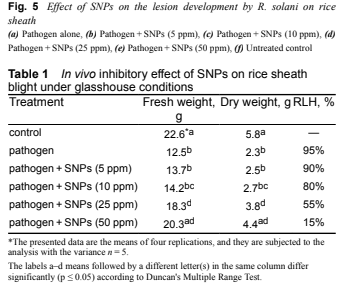
Table 1
3 00Discussion
Silver nanoparticles can denature cells by attacking their membranes and structure. An earlier study found that SNP disrupts transport systems, including ion flow [26]. The dysfunction of ionic flow causes a rapid accumulation of silver ions, disrupting cellular processes such as respiration and metabolism by reaction with molecules. Ji Seon et al. (2009) showed that when treated by spraying SNPs, the mycelium walls were severely damaged and resulted in mycelium decomposition. Considering the cellular effects of silver ions, SNP mediates collapse in Sclerotinia sclerotiorum mycelia with damage to the filament walls [27].
Silver ions are known to inactivate cell enzymes and DNA by coordinating with electron donor groups such as thiols, carboxylates, indols, amides, hydroxyls, etc. [28, 29] According to previous studies of silver nanoparticles, the smaller SNPs, the more Ag + ions they release, affecting microbial activity [30, 31]. As discovered in our investigation, SNP in aqueous small sizes can penetrate the cells of microorganisms and destroy their membrane integrity [32–34]. NPs with a very large surface to volume ratio significantly enhance their membrane permeability compared with non-NPs of the same material [35–37]. NP can penetrate the membrane of microorganisms, leading to cell deformation [38]. NPs, with a large surface-to-volume ratio, exhibit positive antimicrobial properties due to their higher ability to interact with cell membranes through cell wall structural disruption, effects on respiratory chains and differentiation. cell division in DNA and protein as a microorganism [39]. It is likely that the size of the similar SNPs play an important role in their permeability and antifungal activity. In summary, SNPs have an active antifungal activity with excellent bactericidal properties, so they have the potential to be considered an economical and environmentally friendly insecticide. The application of chemical fungicides adds to the potential long-term and indirect costs because it causes dangerous side effects for both human health and the environment [40]. Nature’s editors estimate that any technology takes about 20 years to emerge from the laboratory and become commercialized [41]. The application of nanotechnology in agriculture may take several decades to transition from laboratory to land, but reasonable expectations will be important for this nascent field to flourish [42].
The efficacy of nano-silver was increased by combining antifungal miconazole with nano-silver exhibiting significant fungicidal activity [43]. SNP with chitin inhibited the germination of the spores of the pathogens were investigated [44, 45]. Furthermore, bioactive SNP has been found to control the endogenous fungus of C. gloeosporioides, in vitro [46]. The antifungal activity of SNP was comparable to that of the ionic SNP; however, silver ions are still cytotoxic at the growth inhibitory concentrations of the tested yeasts [47]. According to previous research, both positive and negative effects on plant growth and development have been proposed [48]. In some plant species (e.g. Brassica juncea), SNPs have been shown to affect the total number of plant growth factors including shoot and root length and biochemical properties such as chlorophyll, junction carbohydrate and protein intake and antioxidant enzyme ratio [49].
Conclusion
In vitro and in vivo studies on the antifungal activity of silver nanoparticles at concentrations of 5, 10, 25 and 50 ppm were conducted on the fungal disease R. solani to reduce and prevent blight in rice plants. In vitro results showed that the RHs for mycelia development, sclerotia formation and the germination of sclerotia were (8, 35, 67, 85), (12, 23, 68, 92), respectively. and (15, 26, 57, 98) for their respective SNPs concentration (5, 10, 25 and 50 ppm). The results clearly showed that the RHs were strongly dependent on the SNPs concentration, and basically increased as the SNPs concentration increased. As indicated in in vitro testing, we can conclude an increasing trend in the growth inhibition rate of mycelium, mycelium germination and sclerotia formation with daily SPN counts. increase. By spraying SNPs on rice plants, leaf blight symptoms were reduced and at a concentration of SNPs 50 ppm the disease symptoms completely disappeared. In vivo results showed that the SNP solution created an antibacterial layer around the rice plant to protect the plant from pathogens. It was also shown that SNP greatly affects the formation and germination of sclerotia. SNPs can penetrate fungal cell membranes and cell walls, killing microbial cells. This investigation shows that SNPs can replace chemical pesticides in the control and inhibition of blight, a common rice disease.
Reference source: In vitro and in vivo antifungal properties of silver nanoparticles against Rhizoctonia solani, a common agent of rice sheath blight disease
Meysam Soltani Nejad 1, Gholam Hosein Shahidi Bonjar 1, Mehrdad Khatami 2, Abbas Amini 3, Sonia Aghighi 4,5
1 Department of Plant Pathology, Shahid Bahonar University of Kerman, Kerman, Iran
2 Bam University of Medical Sciences, Bam, Iran
3 Institute for Infrastructure Engineering, University of Western Sydney, Kingswood, NSW 2751, Australia
4 Horticultural Research Institute, Shahid Bahonar University of Kerman, Kerman, Iran
5 School of Veterinary and Life Sciences, Murdoch University, Perth, WA 6150, Australia
NANO NNA VIET NAM
