Grapefruit ( Citrus paradisi ) is a widely cultivated citrus family and its fruit is affected by a variety of biotic and abiotic stresses. Keeping track of the harmful effects of synthetic fungicides , the recent trend is moving towards safer and environmentally friendly fruit disease control. This study aims to diagnose fruit rot of pomelo and prevent it by using green nano zinc oxide (ZnO NPs). Symptoms of fruit rot have been observed in different pomelo growing sites in Pakistan. Disease samples were collected and the pathogen isolated. According to Koch’s postulates, the isolated pathogen was identified as Rhizoctonia solani. For an environmentally friendly control of this disease, zinc oxide nanoparticles were prepared in the seed extract of Trachyspermum ammi and characterized. Fourier transform infrared (FTIR) spectra of these NPs described the presence of stable and reducing compounds such as phenol, aldehyde and vinyl ether, especially thymol (phenol). X-ray diffraction (XRD) analysis revealed their crystal nature and size (48.52 nm). Energy-dispersive X-ray (EDX) analysis thoroughly explained the presence of key elements in the samples, while scanning electron microscopy (SEM) confirmed the morphology of the bio-fabricated NPs. learn. ZnO NPNs exhibited very good antifungal activity and the most significant inhibition of fungal growth was observed at a concentration of 1.0 mg/ml of green NPs, in vitro and in vivo. These findings describe that the bioactive components of T. ammi seed extract can effectively reduce and stabilize ZnO NPs. This is a cost-effective method to successfully control pomelo fruit rot.
Nội dung bài viết
Introduction
Citrus fruits have the highest global production of any other fruit genus, with an estimated production of 95 million tons in 2019–20, including grapefruit, lemons, oranges and tangerines ( Costa et al. , 2020 ). Citrus fruits are very susceptible to various pathogens ( Rasool et al., 2014 ) and these fruit drops are mostly caused by fungal diseases ( El-Otmani et al., 20011 ). Fungal diseases are a growing concern worldwide, with a serious but poorly analyzed impact on public health ( Zhou et al., 2020 ). Food insecurity and malnutrition are major problems in both developing and industrialized countries of the world (Friedmann, 1993; Koffi et al., 2017 ; Saleem et al., 2020a; Saleem et al., 2017). events, 2020b ). Fungi have been reported to cause major yield losses of economically important fruits. Due to poor pre- and post-harvest management strategies, developing countries observed fungal losses of 20–25% of total crop production ( Dukare et al., 2019 ). Under unfavorable environmental conditions, this yield loss can increase to 50% or more ( Carmona-Hernandez et al., 2019 , Saleem et al., 2020c , Saleem et al., 2020d , Saleem et al. events, 2020e ). Fungal diseases can damage all types of plant tissues, at all stages of plant growth ( Fernández-Acero et al., 2007 ). Rhizoctonia solani, Phythophora spp. and Fusariumspp. can infect aerial and terrestrial parts of plants ( Bashir et al., 2018 ). Due to their perishable nature, the fruit is very susceptible to attack and rotting by a variety of fungal diseases ( Chohan et al., 2015 ). Fruit yield loss due to fungal diseases is estimated to be 50% of total fruit production ( Zhang et al., 2017 ).
The use of pesticides in crop production is very important for disease control ( Zhang et al., 2011 ). However, these agricultural practices can be detrimental to humans and the environment, and their potential benefits need to be balanced against these harms ( Donley, 2019 ). Fungicides chemical (insecticides) are widely used on various crops and they are being used as a staple of farming nowadays ( Dara, 2019 ). Excessive and unsafe use of these chemical fungicides has been reported to damage soil fertility ( Joko et al., 2017 ). Contaminated soil eventually leads to loss of growth, yield and symbiotic properties ( Garg et al., 2017). Due to their adverse effects, people have begun to look for alternatives to chemical fungicides. Scientists are emphasizing biologically active natural resources and most higher plants and their components effective in plant disease management. Due to environmental and health related concerns, plant scientists are interested in identifying cheaper and environmentally friendly biological compounds for plant disease control ( Cherkupally et al. , 2017 ).
Nanotechnology is an emerging field of research applied in the fields of chemistry, physics, biology, pharmaceuticals and materials science ( Fahimmunisha et al., 2020 ; Govindarajan and Benelli, 2016 ). Nanotechnology is being applied in the field of plant pathology and it is showing great effectiveness in the treatment of various diseases ( Park et al., 2006 ). The use of nanoparticles as antifungal and antibacterial agents has been proposed as a cost-effective and environmentally safe alternative strategy to treat pathogenic bacteria ( Alam et al., 2019 ). The promising application of nanotechnology in agriculture has opened up new possibilities and prospects. Nanomaterials are used in a variety of applications ranging from plant protection to nutrition and management operations. farm, due to its small size, high surface-to-volume ratio and unique optical properties ( Shang et al., 2019 ).
Compared to organic-based disinfectants and antibacterial agents, ZnO is a metal oxide that is much more stable and lasts longer. The phytochemical preparation of nano zinc oxide ZnO nanoparticles confers significant antibacterial properties ( Khalil et al., 2018 ). The green synthetic ZnO is a biocompatible and well-processed material in nature, making it attractive for biomedical applications ( Petkova et al., 2016 ). Compared with other metal oxides, ZnO is considered to be more stable and safe ( Kim et al., 2020 ). About 550 tons of ZnO NP are produced each year for various applications around the world ( Bondarenko et al., 2013). ZnO NPNs have been reported to be useful in improving soil fertility , crop yield and zinc availability ( Rossi et al., 2019 , Esper-Neto et al., 2020 ). The U.S. Food and Drug Administration has classified ZnO, along with four other zinc compounds , as recognized as safe (GRAS) ( Food and Drug Administration FDA, 2015 ). ZnO nanoparticles are non-toxic and many studies have described their protective role ( Roselli et al., 2003 ). Extracts from various medicinal plants including Pelargonium zonale, Punica granatum, Aegle marmelos, Olea ferrugina, Ruta Tombolen, Hibiscus subariffa, Passiflora caerulea and Berberis vulgaris were used to synthesize ZnO NPs ( Anzabi, 2018 ) et al., 2020 ; Vahidi et al., 2019 ).
Trachyspermum ammi is known for its healing properties and antibacterial activities ( Sharifzadeh et al., 2015 ). The seed of T. ammi is used in traditional medicine and it is famous for its aphrodisiac properties. Its seeds contain thymol , which is used in the treatment of gastrointestinal diseases and bronchial problems. Its root is diuretic in nature and its oil has antifungal and antibacterial properties ( Singh and Singh, 2000 ).
This study is designed to use nanotechnology to synthesize ZnO nanoparticles from the seed extract of T. ammi to control fruit rot in pomelo.
Materials and methods
1. Collection of disease samples from citrus core regions of Pakistan
During 2018–19, a severe fruit rot was observed in the orchards of four citrus growing regions of Pakistan (, Appendix A Supplementary Material ). The National Agricultural Research Center (NARC) Citrus Orchard, Islamabad (33° 40’12.4″ N 73° 07’34.0″ E) was the first zone/site to collect diseased grapefruit samples . From the second region of Sargodha District, samples of diseased grapefruit were collected from three different locations, i.e. Bhulwal (32° 16’57.4″ N 72° 54’14.4″ E), Kot Momin ( 31° 57’39.6″ N 73° 06′ 38.8″ E) and Syal Mor (31° 58’58.2″ N 73° 06’54.8″ E) ( Figure 1 ). Infected rotten pomelos were collected from random trees, at least about 10 m. All samples were collected between November 2018 and January 2019, kept in individually labeled and protective polythene bags.

Figure 1 . Initially, symptoms appear as spots (A), progressing further to complete necrosis of the fruit (B). Fungi were isolated from diseased fruit on SDA medium (C). The mycelium was observed under a microscope at 100 × magnification (D). After transplanting into healthy fruit, disease symptoms appeared slowly at week 1 (E) but progressed rapidly at week 2 leading to necrosis until week 3 (F).
2. Isolation of pathogens from disease samples
To isolate the pathogen, diseased fruit was surface disinfected with 2% sodium hypochlorite solution and washed with distilled water. For the isolation and development of pathogenic pathogens, diseased 4 Discussion , 5 Conclusions and Prospects ,mm) were excised and placed on potato dextrose agar (PDA). Inoculated Petri dishes were covered with parafilm and kept in an incubator at 26 ± 2 °C for 5 days.
3. Check for pathogenicity
To confirm the pathogenicity of each isolated fungus, Koch’s postulates were followed. Fungal plates of approximately 4 mm from 7-day-old isolates were transferred to Czapek culture medium and shaken for 3–4 days. The culture medium was filtered and a specific spore suspension (10 6 conidia ml −1) was maintained. A total of 12 healthy grapes were drilled with a sterile needle (5 mm deep). Of these, six healthy fruits were inoculated with 5 µL of spore suspension and the remaining six were given 5 µL of distilled water (control). The transplanted fruit was wrapped in felt and kept at 25°C. Disease symptoms were observed and compared with those of the field samples. The pathogen was re-isolated on PDA medium from these inoculated fruits and compared with the fungus originally isolated.
4. Identification of isolated fungi by microscopy
Microscopy was performed to study the morphological features of each isolated fungus. The main structural features such as hyphae (septal/non-septal) and reproductive structures (spore sacs and spores) of fungi were observed under a complex microscope ( James and Natalie, 2001 ). For this purpose, a slide containing mycelium was prepared. Using a mounting needle, fragments of young mycelium from the edge of a fresh fungal culture were placed on a slide with one or two drops of lactophenol blue. Avoid creating air bubbles by carefully placing the cover sheet. Slides were examined under a microscope at 10 × and 40 × magnification. The photos were taken with the help of an on-board camera.
5. Molecular identification of fungi
The isolate was also determined by sequence analysis of 18 S. DNA ribosomal ribosomal gen RNA extracted by CTAB technique ( White et al., 1990 ). Nanodrop® was used to assess the quality and quantity of extracted DNA. For rRNA gene amplification, common forward and reverse primers were used. The 20 µL PCR reaction mix consisted of 0.5 µL each primer, 1 L dNTP, 1 µL TaqDNA polymerase, 0.5 L genomic DNA, and 2 µL 10× polymerase buffer. PCR reactions were performed at 94°C for 4 min, followed by 35 rounds of 94°C for 1 min, 58°C for 1 min, and 72°C for 10 min. The final product was sequenced using the Sequence Navigator (version 1.0.1, Applied Biosystems) and explored in the NCBI database (https://www.ncbi.nlm. nih.gov/).
6. Preparation of plant extracts
Extracts from six different plants including Chenopodium album, Callistemon citrius, Magnifera indica, Inert Lawsonia, Cassia fistula and Trachyspermum ammi were prepared for analysis of their antifungal activity. Seeds of T. ammi and leaves of five other plants were thoroughly washed in running water and dried in the shade in the control medium. The dried leaves and seeds were ground into powder, and each sample of 100 g of powder was mixed in 1000 ml of double-distilled water that was autoclaved. The mixtures were placed in a shaking incubator at 24°C for 48 h and boiled for five min before being incubated for 15 min in a water bath at 50°C. The mixture was cooled and filtered through a muslin cloth and No Whatman filter paper. 1. All filtrates were labeled and stored at 4°C, until further use.
7. Analysis of in vitro antifungal activity of plant extracts
Using the tainted food technique, the antifungal activity of six plant extracts was evaluated in vitro. For this purpose, the PDA medium was autoclaved, and the warm culture medium was mixed with 5 µL of each plant extract, separately, and allowed to solidify. PDA medium without plant extracts was used as a control. Each Petri dish was inoculated with a culture dish (5 mm) of isolated fungi and incubated at 25°C. After seven days of incubation, inhibition of mycelial growth was measured by the following formula:Inhibition of growth of fungi % = (CT) / 100Where C = Mean mycelia growth in the positive control, T = Average mycelium growth in treated Petri dishes.
Among the six plant extracts, the seed extract of T. ammi gave the best results and it continued to be used for the synthesis of nanoparticles.
8. Green preparation of nano zinc oxide (ZnO NPs)
According to the methodology of Matinise et al. (2017) , ZnO NPs were synthesized in the seed extract of T. ammi. The seed extract was heated at 60–80 °C and added 2 mg of Zinc Nitrate Hexahydrate [Zn (NO 3 ) 2.6 H 2 O]. The mixture is boiled until a thick yellow paste is formed. This paste was placed in a porcelain crucible and subjected to high temperature at 500°C for 3 h, in a preheated kiln. Due to this intense heating, a pale yellow powder was obtained, indicating the formation of zinc oxide nanoparticles ( Elumalai and Velmurugan, 2015 ). These NPs were further characterized, before analyzing their antifungal activity.
9. Characterization of nano zinc oxide
The following parameters were used to analyze the size, shape and composition of NPs.
9.1. Four Transform Infrared Spectroscopy (FTIR)
The type of functional group binding of the plant extract to the nanoparticles was determined by spectroscopy FTIR . Using the KBr pellet method, 10 mg of nanoparticle powder was packaged in 100 mg of KBr tablets and analyzed in a spectrometer FTIR with a resolution of 4 cm −1 and a scanning range of 400–4000 cm −1 .
9.2. X-ray diffraction (XRD)
The crystalline nature of the newly fabricated green nanoparticles was investigated using an X-ray diffractometer. Finds were drawn from the atomic structure of the powder and solid crystal samples, as well as the angles at which where diffraction occurs. The size of the nanoparticles was determined by the following formula of Scherrer (2018) :D=0.9λ/βcosθD = average crystal domain size perpendicular to the reflection plane, K = form factor, ʎ = X-ray wavelength, β = FWHM (full width at half-maximum), and θ = diffraction angle.
10. Scanning electron microscopy (SEM) and energy dispersive X-ray (EDX)
SEM and EDX analyzes were used to determine the shape, scale and chemical composition of the prepared green nanoparticles. To form a suspension of green zinc oxide nanoparticles in distilled water, the samples were soaked for five min. A drop of suspension was mounted on conductive tape with a double carbon coating and allowed to dry under a lamp. On the VEGA3 TESCAN instrument, SEM and EDX analyzes were performed.
11. Antifungal activity analysis of zinc oxide nanoparticles, in vitro
To analyze the in vitro antifungal activity, zinc oxide nanoparticles were added to PDA medium at different concentrations. With the help of the sheath borer, 4 mm culture plates of isolated fungi were placed in the center of the PDA petri dish. Green nanoparticle-free culture medium was used as a positive control. Inoculated Petri dishes were placed in an incubator at 25 ± 1 °C and fungi were allowed to grow. After one week, growth inhibition in each Petri dish was measured using the following formula: Fungal growth inhibition % = (CT) * 100 where C = Mean mycelia growth in the active control and T = Average mycelium growth in treated Petri dishes.
12. Comparative in vivo antifungal activity analysis of nano zinc oxide and plant extracts
The antifungal activity of nano zinc oxide and six plant extracts was also tested, in vivo. Selected healthy pomelos were inoculated with isolated fungi, according to the standard “wound inoculation method”. For this purpose, six healthy fruits were wound with a needle and inoculated with 10 µL of spore suspension (10 6 spores/ml) of the fungus. Pathogens were allowed to enter and infect and after two days of inoculation, the above-mentioned nanopesticides were sprayed (until watery) on three randomly selected fruits. Three fruits were left untreated (control). All fruit is covered with a muslin cloth that has been autoclaved to avoid any contamination. Following the same method, 10 µL of each selected plant extract was sprayed (until watery) on three randomly selected fruits to control fruit rot, replacing the nanofungicides. After seven days of inoculation, the diseased area of each fruit was measured to evaluate the effectiveness of the individual pesticides..
RESULT
Isolation and characterization of pathogens from grapes
Disease symptoms were observed in fruit samples, in the field ( Figures 1 A and B). The pathogen was successfully isolated and off-white mycelial colonies were observed on the PDA ( Figure 1 C). Microscopic observation allowed us to see the morphology of the fungus and could easily observe the long, rod-like hyphae ( Figure 1 D). All these characteristics identified this pathogen as Rhizoctonia solani (Parmeter, 1970). The pathogenicity of R. solani isolates was effectively realized. After four days of implantation , initial symptoms can be observed as small dark brown spots ( Figure 1E). These nodules progressed rapidly by week 2 and led to complete necrosis by week 3 ( Figure 1 F). The fungus was again isolated from these diseased fruits and found to be similar to the cultured fungus. These findings confirmed the virulence and involvement of this fungus in pomelo fruit rot. Successful sequencing of the amplified 18 S rDNA region of the isolated pathogen also confirmed its identification. BLAST analysis of the resulting sequences showed >99% similarity with R. solani (HG934415.1).
Antifungal activity analysis and selection of plant extracts for the synthesis of nano zinc oxide ZnO . NPs
Among the six native medicinal plants tested, the seed extract of T. ammi exhibited the best antifungal activity ( Table 1 ). Based on these results, seed extracts of T. ammi were used to synthesize ZnO NPs.

Table 1 . In vitro antifungal activity of six indigenous R. solani medicinal plant extracts.
Characterization of nano zinc oxide ZnO NPs synthesized in seed extracts of T. ammi
The following parameters have significantly helped us characterize nano zinc oxide and suggest their application for antifungal activity analyses.
FT-IR spectra of green ZnO NPs
FTIR analysis of nano zinc oxide, prepared in the T.ammi extract showed a characteristic peak at 1738.69 cm −1 , indicating the presence of the Aldehyde (C=O stretched) group on the NP ( Figure 2 ). The spectrum of this sample also shows some specific bands at 1366.16.34 cm -1 (OH bending) and 1216.90 cm -1 (CO stretching), indicating that the organic components of the extracts are resistant to responsible for the successful preparation of ZnO NPs. The prepared NPs were found to contain biomolecules such as phenol (thymol), aldehyde and vinyl ether. Thymol has been reported to be a reducing agent and plays an important role in NP biosynthesis ( Manukumar et al., 2017).
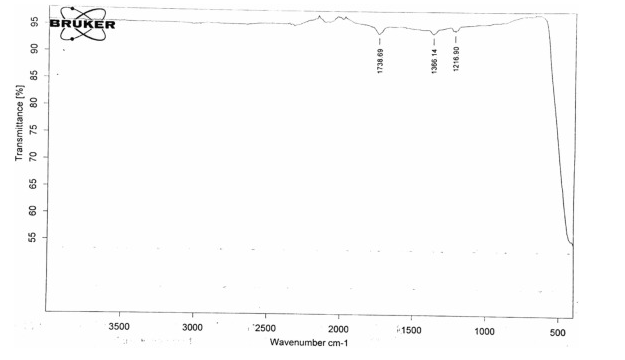
Figure 2 . The FTIR spectrum shows sharp peaks of ZnO NPs synthesized in T. ammi.
XRD analysis of the synthesized nano zinc oxide ZnO . NPs
The X-ray diffraction pattern of ZnO NPs shows remarkable peaks at 31.8 0 , 34.32 0 , 47.44 0 , 56.44 0 , 62.80 0 , 66.25 0 , 67.80 0 , 68.93 0 , 72.51 0 and 76.86 0 for peak values (100), ( 002), (101), (102), (110), (103), (200) , (112), (201), (004) and (202) ( Figure 3). The planes of XRD samples had a great deal with JCPDS number 01-079-0207. The prepared NP sample was indexed into a single-phase hexagonal structure with space group number P63mc:186, zinc oxide. The average particle size (48.52 nm) was calculated using the Debye Scherrer formula, which is consistent with previous findings ( Saravanakkumar et al., 2016 ).

Figure 3 . XRD analysis of ZnO NPs synthesized in T. ammi .
SEM and EDX analysis of nano zinc oxide ZnO . NPs
The EDX spectra of NPs showed the predominant presence of zinc (43.72%), carbon (30.12%) and oxygen (26.12%) ( Fig. 4 A). EDX analysis also shows that the optical absorption peaks of the NPs show their surface plasmon resonance effects . The origin of these elements lies in the composition of the phyto extract ( Wei et al., 2009 ).

Figure 4 . EDX (A) spectra and SEM images (B) of ZnO NPs prepared in T. ammi.
SEM analysis helped us to investigate the superficial morphology of ZnO NPs, which were prepared in the T. ammi extract. SEM images show the hexagonal shape of the aggregated NPs, which are arranged in an upward direction to form aggregates of bundles ( Figure 4 B).
Antifungal activity of zinc oxide nanoparticles, in vitro and in vivo
The synthesized zinc oxide nanoparticles exhibited significant growth inhibition of R. solani at concentrations of 1.0, 0.75 and 0.5 mg/ml ( Table 2 and Appendix 2). Lower concentrations of NPs (0.25 and 0.1 mg/ml) showed that the tested fungus inhibited growth the least. In in vivo treatment, the fungicide nano zinc oxide showed good disease control (Table 3 and Appendix 3). NPs control the disease greatly and the antifungal activity of these zinc oxide nanoparticles at the concentration of 1.0 mg/ml is better than all the plant extracts. The significant growth inhibition describes the importance and possible use of these NPs in disease control.
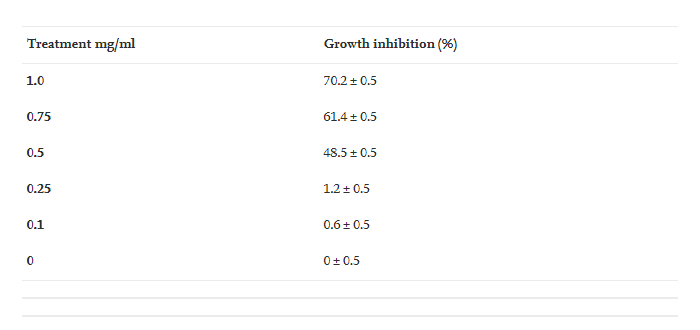
Table 2 . Growth inhibition of R. solani ZnO NPs at different concentrations of mg/ml.

Table 3 . Diseased area on grapefruit after treatment with different concentrations of ZnO NPs.
DISCUSS
This study described the successful isolation and characterization of fruit rot fungi and their environmentally friendly control using nanoparticles. In this study, R. solani was found to be associated with fruit rot of grapefruit in Pakistan. Control of these diseases is a matter of great interest to today’s plant pathologists. For better human health, the use of chemical pesticides is being discouraged in the civilized world. Scientists are focusing on the use of natural antibacterial products, especially those of plant origin.
In this study, we used six reputable and well-documented native plants to test their antifungal abilities. Among them, the T. ammi seed extract with the best antifungal activity was used to synthesize the green color of NPs. The participation of alkaloids and flavonoids, which are known to be active biological compounds against fungi and bacteria, may indicate the antifungal effect of T. ammi ( Avita, 2013 ). Individually or in combination, the bioactive polyphenol compounds found in the seed extracts interfere with the life cycle of fungi by binding to their protein molecules, acting as chelating agents, altering structural composition synthesis, weakening or destroying the permeability barrier of cell membranes, and altering the physiological state of cells ( Rongai et al., 2015 ). Previous studies have also demonstrated antifungal activity of T. ammi against many fungal strains due to the presence of multiple antifungal compounds in the seed extract of T. ammi ( Jyoti et al., 2019 ). The FTIR spectra of the prepared NPs revealed the presence of thymol , a well-known antibacterial phenolic compound ( Khan, 2017 ). Previous studies also described that bioactive compounds of plant extracts act as key players and reduce metal ions to generate NPs. This combination becomes more lethal to pathogens and controls disease, more effectively ( Chouhan and Meena, 2015 ). T. ammi leaf extract has also been used to fabricate green nanoparticles of copper, silver, nickel and magnesium ( Jagana et al., 2017 ).
In this study, the synthesis of green zinc oxide nanoparticles was successfully performed using seed extract. Studies describe that bioactive compounds present in the seed extracts of T. ammi can be adsorbed on the surface of metal nanoparticles by possible interaction of functional groups and compounds. These substances act as reducing and stabilizing agents, during the formation of NPs. Plants are rich in compounds such as amino acids, polyphenols, nitrogenous bases, and reducing sugars. Such chemicals act as reducing and stabilizing agents, in the synthesis of magnetit nanoparticles ( López and Antuch, 2020). In this study, the prepared green zinc oxide nanosheets showed enhanced antifungal activity because of their small size and stability. The smaller size increases the dispersion and penetration of the intracellular matrix, while also interfering with intracellular Ca 2+ absorption and causing cell damage ( Srihasam et al., 2020 ). Binding of NPs to microbial cell membranes leads to damage to cell membranes and intracellular organelles ( Basak et al., 2014 ).
ZnO NPNs are environmentally friendly, non-toxic, biosafe and biocompatible ( Mohammad et al., 2010 ). The U.S. Food and Drug Administration has listed ZnO, along with four other “zinc compounds” as “generally recognized as safe (GRAS)” ( Food and Drug Administration FDA, 2015) ). Previous studies have described that ZnO is not toxic, if used at low concentrations. ZnO NPs can sometimes adversely affect living systems if hepatocytes are exposed to concentrations of 14–20 mg/ml for 12 h ( Siddiqi et al., 2018 ). It also causes DNA damage due to oxidative stress. Antibacterial agent made of zinc oxide nanoparticles (ZnO NP) has emerged as a new type. The ZnO NPs first meet the bacterial cell and adhere to the outer surface of the plasma membrane. The structure of the plasma membrane is disrupted and its permeability is affected due to this interaction. Disruption of membrane structure and subsequent accumulation of ZnO NPs in the cytoplasm impede basic cell developmental processes ( Zhang et al., 2017 ). ZnO NPs mediate hydrogen peroxide, which is one of the most important antimicrobial agents ( Sawai et al., 1998 ). In addition, NPs of ZnO contain other reactive oxygen species , such as hydroxyl radicals and singlet oxygen, which stimulate cell death.
Conclusion and prospects
The use of plant extracts for the preparation of NPs is an easy and cost-effective method. These NPs are environmentally friendly and non-toxic. Plant biomolecules such as proteins (enzymes), amino acids, polysaccharid , alkaloids, alcohol compounds and vitamins can play a role in NP reduction, formation and stabilization. Fungal growth can be inhibited by zinc oxide nanoparticles. Nanotechnology may have agricultural solutions and could revolutionize current disease management systems. Materials scientists and biologists need to work together to deepen their understanding of the underlying mechanisms of interaction in a complex biological nanosystem. A detailed understanding of the structural properties of nanoparticles, such as morphology, scale, functional groups and active adsorption/loading capacities, can provide a useful guide as a point of reference. starting point for the rational selection of suitable nanoparticles. It is also important to select a robust and reproducible system for conducting biocompatibility and efficacy studies at the cellular, organismal and pest ecosystem levels, in order to achieve conditions as close to the field as possible.
Reference Source: Antifungal activity of Zinc nitrate derived nano Zno fungicide synthesized from Trachyspermum ammi to control fruit rot disease of grapefruit Musrat Ali a Xiukang Wang b Urooj Haroon a Hassan Javed Chaudhary a Asif Kamal a Qurban Ali c Muhammad Hamzah Saleem d Kamal Usman e Aishah Alatawi f Shafaqat Ali g h Muhammad Farooq Hussain Munis a
NANO NNA VIET NAM
